The Reaction of Butane With Oxygen Is Called
The hydrogen ensures that the resulting alkenes and cycloalkenes subsequently react with hydrogen to form saturated compounds. If there isnt enough oxygen butane will undergo incomplete combustion.

Complete Combustion Of Butane C4h10 Balanced Equation Youtube
In organic chemistry an alkane or paraffin a historical trivial name that also has other meanings is an acyclic saturated hydrocarbonIn other words an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carboncarbon bonds are single.

. This property is called isomerism. C12H22O11 11 H2SO4 12 C 11 H2SO4 11 H2O How do you know the concentrated sulfuric acid is a catalyst. The reaction of proane and oxygen occurs under the high pressures of both propane and oxygen.
What is the reducing agent in this reaction. 1-butane butanol 1-butanol 4-butanol. Its ionic formula is written as CaCl 2 the neutral combination of these ions.
When we count the shared. In fact if combustion is complete the products will be carbon dioxide and water and of course heat is given off the reaction is highly exothermic which is ordinarily the purpose of this reaction. Is the concentrated H2SO4 a catalyst in this reaction.
Methanation Unit Even small quantities of CO 01 vol. Chemical Reaction Engineering 3rd Edition by Octave Levenspiel PDF Chemical Reaction Engineering 3rd Edition by Octave Levenspiel 성택 김 - Academiaedu Academiaedu no longer supports Internet Explorer. The purified gas with about 01 vol CO2 is called synthesis gas.
Each oxygen also has 2 non-bonding pairs of electrons. Name the following compound. Oxidation of propane to acrolein and ammoxidafion to acrylonitrile with molecular oxygen proceed over complex metal oxide catalysts.
Acids are defined as compounds that donate a hydrogen ion H to another compound called a baseTraditionally an acid from the Latin acidus or acere meaning sour was any chemical compound that when dissolved in water gives a solution with a hydrogen ion activity greater than in pure water ie. A pH less than 70. Combustion takes place at an elevated temperature.
Eg- Butane C 4 H 10 has 2 isomers. The main reaction occurring in the reformer is the conversion of methane to a mixture of CO. If there is enough oxygen butane undergoes complete combustion.
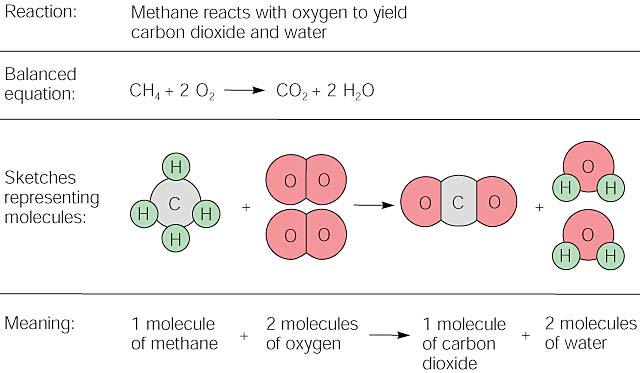
This is because butane is highly flammable. It usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. In both cases combustion produces heat energy.
The products released are often gaseous and the resultant mixture is commonly called smoke. Molecular Oxygen Molecular oxygen makes up nearly 21 of the atmosphere. Two chloride ions were needed in the final compound because.
Those ions are stable since they have filled valence shells. The atomic number of oxygen is 8 its EC is 26 it has 6 VE it needs 2 electrons more to attain stability. Eg -Ethyl ethanoate reacts with sodium hydroxide to form sodium acetate.
Note that it is assumed that the nitrogen will not normally undergo any chemical reaction. Abstraction produces a new radical and a new spin-paired molecule. The selective formations of acrolein and acrylonitrile also required high reaction temperature around 500C.
Hydrogen abstraction describes when a hydrogen atom is removed from a hydrogen donor molecule eg. Butane can be used as an enhancer for the volatilization of gasoline. A combustion reaction is a major class of chemical reactions commonly referred to as burning In the most general sense combustion involves a reaction between any combustible material and an oxidizer to form an oxidized product.
It is composed of Ca 2 cations and Cl anions. A combustion reaction is defined as a highly exothermic redox reaction that involves the reaction with oxygen gas. In this example butane is dehydrogenated to butene.
Good signs that youre. This is different from homolysis which results in two radicals from a single spin-paired molecule and doesnt include a radical as its reactant. O2 CONV OXYGEN O2 C2H6 CONV ETHANE C2H6 C3H8 CONV PROPANE C3H8 N-BUTANE CONV N-BUTANE C4H10.
The species that is oxidized is called the reducing agent because it gives up an electron so that another species can gain an electron be reduced. Bases are the chemical opposite of acids. Tin or silicon hydride with its one electron.
The simple electron dot diagram indicates that O 2 has a double bond one sigma bond and one pi bond between the two oxygen atoms. The nitrogen is called the nitrogen cycle. The Combustion Process - The basic combustion process can be described by the fuel the hydrocarbon plus oxydizer air or oxygen called the Reactants which undergo a chemical process while releasing heat to form the Products of combustion such that.
They are Normal butane and Iso butane. The products of combustion are called oxides. Alkanes have the general chemical formula C n H 2n2The alkanes range in complexity from.
If there is enough oxygen the combustion will be complete ie the products of the combustion will not burn. For example in the reaction of calcium and chlorine the compound is called calcium chloride. Figure 10 A mechanism for the reforming of butane to 2-methylpropene isobutene.
It is a heat releasing exothermic redox chemical reaction that usually occurs between a fuel and oxidizing agent mostly oxygen of the atmosphere. H 2 The species that is reduced is called the oxidizing agent because it takes an electron away from another group raising that groups oxidation number. Butane can be burnt in the presence of oxygen.
Because platinum is involved the reforming is sometimes called platforming. This reaction is called.

Solved The Reaction Of Butane With Oxygen Is Called Addition Chegg Com

How To Balance C4h10 O2 Co2 H2o Butane Combustion Reaction Youtube

Solved 11 The Reaction Of Butane With Oxygen Is Called Chegg Com
No comments for "The Reaction of Butane With Oxygen Is Called"
Post a Comment